GIỚI THIỆU TopSENSI ® HBV qPCR Kit
Hepatis B Virus is a member of the Hepadnaviridae family of viruses with a double-stranded DNA structure. It is estimated that 350 million people are chronically infected with HBV each year. Patients with chronic hepatitis can develop cirrhosis and liver cancer. Therefore, accurate detection and quantification of HBV in blood will make treatment easier for the patients.
TopSENSI ® HBV qPCR Kit This is an HBV testing kit based on Real-time PCR method, enabling precise detection and quantification of HBV concentration in human serum or plasma samples. The kit includes a set of 5 standards, allowing for the construction of a standard curve to accurately determine the initial target agent quantity present in the test sample. Additionally, the exogenous internal controls within the HBV diagnostic kit can monitor the extraction process or master mix, ensuring accurate results with high sensitivity and specificity.
HIGHLIGHTS
- Simple process easy manipulation
- The time to perform PCR is fast, only about 1 hour and 30 minutes
- High specificity, sensitivity (Detectability up to 25 IU/mL)
- Taqman probe technology
SPECIFICATIONS
| Target | Quantify Hepatitis B Virus (HBV) |
| Input sample | Serum/plasma samples |
| Input sample volume | 200µL |
| DNA volume | 10µL |
| Color channel detection | FAM: detect HBV HEX: internal control |
| Analytical sensitivity | 25 IU/ mL |
| Technology | TaqMan probe |
| Standard curve | (E1: 10¹) (E2: 10²) (E3: 10³) (E4: 10⁴)(E5: 10⁵) |
| PCR time | 1h30m |
| Specificity | Detect HBV A-H types |
| Component | HBV qPCR mix, HBV standard ( E1, E2,E3,E4,E5), Negative control, Internal control, Tube PCR. |
| Storage | 12 months at -20oC |
Recommended for extraction kit
| Automatic extraction | HI-512 | TopPURE® Maga Serum DNA/RNA Extraction Kit |
| Silica column | HI-332 | TopPURE® Serum Viral Extraction Kit |
DATA EXPERIMENT
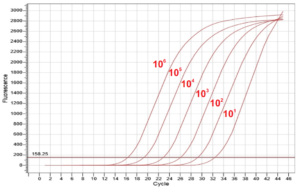
Figure 1Real-time PCR results to assess the sensitivity of the HBV test kit (ABT)

Figure 2:Real-time PCR results evaluate the repeatability of the HBV test kit (ABT)
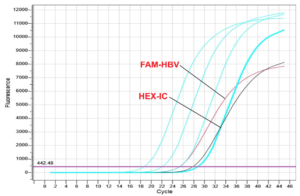
Figure 3:Real-time PCR results of patients infected with Hepatitis B virus.

Figure 4: Results of quality assessment between ABT kit and R supplier's kit on HBV-positive patient sample 86. The results show that the TopSENSI ® HBV qPCR Kit has quality equivalent to R (Evaluated on the same reference system)

Figure 5:Results of stadard samples in HBV quantitative by using Real-time PCR.
Read more: Video






Reviews
There are no reviews yet.